ORLANDO, Florida — Achieving target glucose and lipid levels reduces the risk for diabetic retinopathy (DR) and other adverse health outcomes in people with diabetes, but all are associated with a greater risk of developing age-related macular degeneration (AMD), new research suggested.
In two cohorts with type 1 diabetes, the presence of AMD was associated with less severe DR and diabetic macular edema (DME), while milder DR at baseline was associated with an increased risk for AMD. Measures of metabolic health, including insulin sensitivity, glucose, and triglyceride/high-density lipoprotein ratio, were also inversely associated with AMD.
The findings were presented on June 23, 2024, at the annual American Diabetes Association (ADA) 84th Scientific Sessions by Ward Fickweiler, MD, a clinical fellow in ophthalmology at the Joslin Diabetes Center and Harvard Medical School, Boston.
"We're seeing an opposite association between AMD and DR severity. These are very common sight-threatening diseases. The underlying mechanisms for advanced stages of both diseases have been described as similar, but we're seeing an opposite relationship," Fickweiler told Medscape Medical News.
The reasons for the paradox aren't clear, "but we think it might have to do with glucose and lipid metabolism," he said, adding that while this shouldn't discourage people with diabetes from controlling their blood sugar, "I do think future studies are urgently needed to investigate this interaction."
Previous epidemiologic studies have produced mixed results as to whether diabetes is a risk factor for AMD. Most of these studies focused on type 2 diabetes and haven't assessed whether the effect might be cumulative or protective. This is the first study to examine the relationship between DR severity and AMD, he noted.
"Given the increasing prevalence of diabetes in the elderly, understanding factors that influence age-related macular degeneration and diabetic retinopathy is crucial to target intervention for both," Fickweiler said in his presentation.
Asked to comment, Thomas W. Gardner, MD, professor of ophthalmology and physical sciences and internal medicine at the University of Michigan, Ann Arbor, told Medscape Medical News, "I think there's a strong association, but the causality is another question…It's just an initial observation that needs a lot more work, so I don't think doctors should be trying to do anything about it in terms of counseling patients or treating…We fundamentally don't know what causes macular degeneration."
Gardner also pointed out that the first cohort examined in the study, the "Joslin 50-Year Medalists" who have lived with type 1 diabetes for 50 or more years, may differ from other cohorts with diabetes. "The medalists are very special because by definition they're survivors. What has allowed them to be survivors during a period of time when diabetes control was not very good? They may have some familial survival factors that has allowed them to be relatively healthy. And, maybe that just happens to be associated with macular degeneration."
While little can be done currently to prevent the development of AMD, the Age-Related Eye Disease Studies dietary supplements may help slow AMD progression for some individuals. Also, smoking accelerates AMD, so those who smoke should be encouraged to quit, Gardner noted.
Inverse Relationship With AMD Seen With DR, Cardiometabolic Metrics
Of the 582 Joslin 50-Year Medalists, 41.6% had proliferative DR (PDR), and 43.1% had no or only mild DR, while just 15.3% had moderate to severe non-PDR. Those proportions were similar in a second cohort of 1413 people with type 1 diabetes of shorter duration from Joslin's Beetham Eye Institute (BEI), with 46.0% having no or mild DR and 35.1% having PDR.
The two groups were similar in age, both about 65 years old. The Medalist group had been diagnosed with type 1 diabetes considerably younger, average 10.7 years, vs 20.1 years for the BEI group.
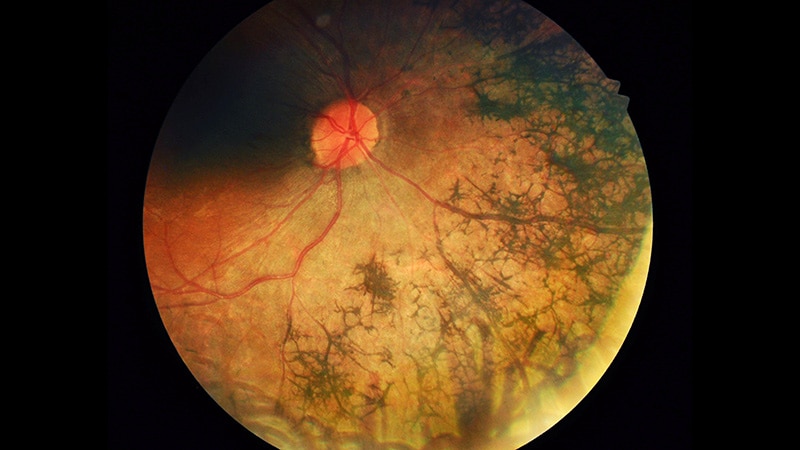
Among 304 of the Medalists with longitudinal follow-up, the prevalence of AMD at baseline was 8.1%, in the range seen in the general age- and race-matched population. The severity of DR was associated with a lower prevalence of AMD, from just 4.8% in 125 with PDR to 10.0% in 33 with moderate to severe DR to 19.5% in 146 with no or just mild DR. The level of AMD, from 1 to 4, also was also inversely related to DR severity.
At a follow-up Medalist visit at a mean of 5 years after the baseline Medalist visit, there was an expected increase in AMD prevalence and a similar inverse association between DR severity and early vs late stages of AMD. Overall, the proportions with AMD at this point were 41.1%, 32.6%, and 18.2% for no-mild, moderate to severe, and PDR, respectively, all significant differences. Fickweiler reported.
Because of the potential for bias in the Medalist group, the investigators did the same analysis of the 1413 age-matched people in the BEI cohort who had type 1 diabetes of shorter duration. Again, there was an inverse association between the presence of AMD and DR severity at the baseline visit, replicating the Medalist findings.
At baseline, AMD was present at baseline in 13.6%, 10.5%, and 3.3% of those with no-mild, moderate to severe, or PDR, respectively. At 5-year follow-up, those proportions were 14.5%, 8.2%, and 3.0%, "which confirms our previous findings," Fickweiler said.
Moreover, in the BEI cohort, the presence of AMD was associated with a lower likelihood of DME, 16.7% of those with no AMD vs 7.6% of those with AMD (P = .007).
Certain genetic variants known to be associated with AMD were associated with AMD in the Medalists, but none of those variants were associated with DR severity.
Among 150 Medalists who had continuous glucose monitoring data, time above range was positively associated with the presence of PDR, and time below range was negatively associated with PDR. In contrast, time below range was positively associated with AMD (P = .045).
After adjustment for age and kidney function, higher triglycerides, triglyceride/high-density lipoprotein ratio, and lower systemic insulin sensitivity were all associated with greater DR severity, but inversely with AMD. Similarly, for advanced glycation end products, measured in a subset of Medalists, the association was positive with DR (P = .02) and negative with AMD (P = .05).
"These findings suggest that metabolic controls of hyperglycemia and lipidemia can delay DR but may worsen the progression of AMD. The inverse risk of AMD and DR suggests accelerated aging is not likely a major cause of DR," Fickweiler concluded.
Fickweiler and Gardner had no disclosures.
Miriam E. Tucker is a freelance journalist based in the Washington, DC, area. She is a regular contributor to Medscape Medical News, with other work appearing in the Washington Post, NPR's Shots blog, and Diatribe. She is on X @MiriamETucker.

.webp) 2 days ago
5
2 days ago
5























 English (US)
English (US)